Since the problem of blackbody radiation directly led to the beginning of the old quantum theory, we also emphasized, why do people study blackbody radiation? And what is blackbody radiation?
Today’s topic is, how does Planck, the founder of quantum theory, solve the problem of black body radiation?
In the 1890s, at the Imperial College in the suburbs of Berlin, there was an optical laboratory. This laboratory was set up specifically to overcome the problem of black body radiation. The leader of the laboratory was Otto Lummel, and he had one The 29-year-old young man named William Wien; Wien first made a crucial breakthrough in the issue of blackbody radiation.
At this time, the optical laboratory has been able to more accurately measure the energy distribution curve of the black body radiation.
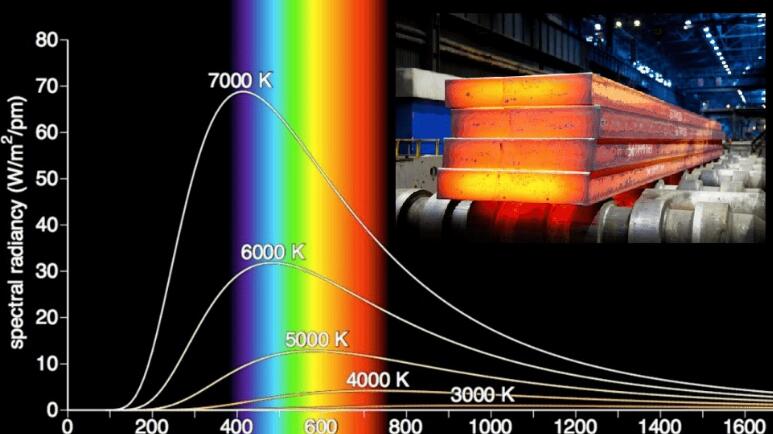
The picture we see now is the curve of the blackbody radiation energy distribution. The abscissa represents the wavelength, the unit is nanometers, the wavelength range of visible light is between 400 and 700, and the vertical axis is the radiation density, which can also be called the radiation intensity.
The lines of different colors represent the curve of the blackbody energy distribution at different temperatures. It can be easily seen that the radiation intensity will increase with the increase of the wavelength. After reaching a peak, then it will begin to decrease. The whole curve is like a bell, so the curve of the black body radiation intensity is also called a bell curve.
The higher the temperature, the more prominent the curve and the more like a bell.
Because Wien can obtain first-hand experimental data in the laboratory, he proposed a displacement law to describe the black body radiation curve at different temperatures in February 1893, and gave a simple mathematical formula .
The law that Wien discovered is relatively simple. It is said that as the temperature of the black body increases, the peak of the black body radiation intensity will move toward the short wave, that is, the top of the bell curve moves toward the short wave, and the wavelength of the peak is multiplied by the temperature. constant.
This constant is easy to calculate. You only need to measure the wavelength of the blackbody’s radiation peak at a certain temperature to calculate this constant. Through this constant, we can know the wavelength of the radiation peak of an object at different temperatures.
Wien’s law of displacement explains well why the color of an object changes from red, to yellow, and then to blue-white as the temperature increases.
Three years later, in 1896, Wien made persistent efforts and proposed a mathematical formula to describe the blackbody temperature and radiation intensity distribution. This is the famous Wien formula.
In June of the same year, when Wien published the law of energy distribution, he left Imperial College and went to RWTH Aachen University, where he was promoted from a laboratory assistant to an assistant professor.
This is a career path that every physicist must go through. Follow your supervisor as an assistant for several years, complete your doctoral dissertation, get a doctorate, and then stay in school or go to other universities to be a supernumerary lecturer, or you can be lucky. Be an assistant professor and then be promoted to a full-time professor. At the step of being a full-time professor, you are considered a civil servant.
Closer to home, let’s talk about the issue of blackbody radiation.
As soon as Wien’s law of energy distribution came out, the next task was to measure whether the formula met the experimental data. At that time, the electric heating black bodies used in major laboratories did not have a unified standard, they were all manufactured by themselves, and the temperature of each experiment The measured wavelength ranges are different, so it took four years to verify Wien’s formula.
Some scientists, such as Paschen (the Paschen series in the hydrogen atom spectrum, are named after him). After four years of testing, they say that Wien’s formula is okay, while Lummer and Prinscher of Imperial College London Mu also tested for four years, and always found that Wien’s formula was in the long wave range, and there was an error with the experimental data.
The person who settled this dispute was Planck’s friend Heinrich Rubens. In 1900, the 35-year-old Rubens had joined a full-time professor at the Technical University of Berlin. In late September, Rubens and Kurbo In the temperature range of 200 to 1500 degrees Celsius, the Wien formula was tested for the wavelength range of 0.03 to 0.06 mm, which is 30,000 to 60,000 nanometers, and it was found that the Wien formula did not conform to the long-wave radiation.
On October 7, Rubens brought his wife to Planck’s house for dinner, and by the way told Planck the results of the experiment, and told Planck that in the long wave range where the Wien formula failed, the experimental results showed that, The intensity of radiation is directly proportional to temperature.
On the day Rubens left, it is estimated that Planck did not sleep well all night. Because he did a great thing that can make his name go down in history.
Planck was born in Kiel, Germany on April 23, 1858. His great-grandfather and grandfather were both famous theologians, and his father was a law professor at the University of Munich. He was influenced by his childhood culture and a superior living environment. After many children from ordinary families, when they grow up, others like to go to bars, while Planck likes to go to theaters and concert halls.
After graduating from high school, Planck once wandered between musicians and physics for a long time. After all, people played a good piano, at least it was more beautiful than Einstein’s violin. In 1974, Planck. Kr chose to study physics at the University of Munich, and then transferred to the University of Berlin in 1977.
At the University of Berlin, he had talked to two top German physicists Kirchhoff and Helmholtz at the time, but Planck didn’t like the two lecture styles, which were monotonous and boring. Planck’s later years When I recalled, especially Helmholtz had never prepared a lesson well.
By chance, Planck came into contact with the 56-year-old Clausius. He was a professor at the University of Bonn in Germany. Clausius’ research field is thermodynamics. It was the first time that he proposed the concept of entropy. Planck not only I was attracted by the professor’s lecture style, and after reading Clausius’s thesis on thermodynamics, I was also deeply attracted by the two laws of thermodynamics.
Like Clausius, Planck also believes that the second law of thermodynamics, like the first law, is absolute and universal. In other words, Planck believes that entropy increases whenever, wherever, and energy The law of conservation is absolutely valid.
After staying in Berlin for a year, Planck returned to the University of Munich. His doctoral thesis was about thermodynamics. Before studying blackbody radiation, Planck’s main direction was also thermodynamics.
In 1880, Planck became a non-staff lecturer in Munich. He has been working for 5 years. The non-staff lecturer is a temporary position. Planck collapsed a bit and felt that his future was a little dark.
In 1885, the 27-year-old Planck unexpectedly received an invitation to the position of assistant professor at Keele University, but he suspected that his father had asked him to find a job.
Planck accidentally won the grand prize in 1888, his former mentor Kirchhoff passed away, and the position of full-time professor at the University of Berlin was vacant. The University of Berlin invited Hertz, but Hertz directly refused, and invited Boltzmann again. When Boltzmann saw that Hertz was not going, he didn’t go either. At this moment Helmholtz thought of his student Planck.
In fact, Planck did not have a deep friendship with Helmholtz, but Planck once publicly supported Helmholtz’s point of view at a debate at the University of Göttingen, which gave Helmholz I am impressed.
Since then, Plank has been on the same footing and his career has been smooth sailing, especially in 1894, when Helmholtz and Comte died one after another. Planck suddenly discovered that 36-year-old himself had not done any amazing results yet, unexpectedly unexpectedly. Zhong became a senior physicist at a top university in Germany.
He also served as a theoretical physics consultant for the top academic journal “Physics Yearbook” in Germany.
It may be destined that Planck is not a general in this life. On the night when his friend Rubens left, Planck decided to try to figure out a correct formula first. Now he has three important messages in front of him:
Wien’s displacement law is correct, Wien’s formula is correct in the short wave range, and Wien’s formula is incorrect in the long wave range where the radiant energy is proportional to temperature.
Based on this information, Planck came up with a formula that night and sent a letter to Rubens overnight, asking Rubens to help him test it. A few days later, Rubens came to Planck with the answer. At home, tell Planck that your formula exactly matches the experimental data.
On October 19th, the regular meeting of German physicists was held as usual. At the meeting, Planck wrote his formula on the blackboard, saying that this formula is a slight improvement on the Wien formula, at least it seems to be correct for now; Ke didn’t say much about the physical meaning of this formula, nor did he explain too much. His colleagues also nodded politely.
A few days later, all the laboratories confirmed that Planck’s formula was okay, but for Planck, the things that really bothered him had just begun.
In the next few weeks, Planck had trouble sleeping and eating. Planck recalled in his later years that those few weeks were the most stressful period of his life.
Because Planck, as a theoretical physicist, you only give formulas and do not explain physics. This inevitably makes people feel that you are a bit opportunistic, and even make people suspect that you are relying on luck.
So Planck had to give his formula a meaning in physics, and he had to be ahead of others, but Planck found that his formula could not be explained in terms of classical electromagnetism and classical thermodynamics. I tried many ways to no avail.
In desperation, Planck introduced Boltzmann’s concepts of statistical mechanics and entropy, which he had always disliked. Here is a little bit. Planck did not believe in the existence of atoms at this time, and he did not like Boltzmann. Mann’s explanation of the probability of entropy increase has been mentioned above. He inherited Clausius’ idea that the entropy increase is absolute and there is no probability.
Boltzmann’s statistical entropy means that there is a certain probability that the entropy will decrease. For example, if you heat up a rock on the ground, it may jump into the air again, and the broken glass may recover. The air may suddenly all move to a corner and so on, but the probability of this situation is very low and basically impossible.
Planck thinks this statement is extremely absurd. Entropy increase does not require probability, but Planck can no longer take care of so much in order to solve his own dilemma. He can only try to accept Boltzmann’s physics.
And imagine the inner wall of the black body cavity as a huge array of oscillators. Each oscillator is a simple harmonic oscillator with a fixed vibration frequency. The oscillator of each frequency can only emit radiation of the corresponding frequency, for example, one The vibration frequency of the vibrator is V, so it can only emit radiation with frequency V.
Then the frequencies released by all the oscillators are added together to release the frequencies of the entire black body radiation.
When Planck allocated the energy of the black body to each oscillator, he found that only the oscillator releases energy one part at a time, and the energy that the oscillator has can only be this one. His equation can only be established when the energy is an integer multiple.
The minimum energy of each part is the frequency V of the oscillator multiplied by a constant h. This constant is the Planck constant, which is 6.626 times 10 to the minus 37 power, erg seconds.
We know that when the oscillator frequency is constant, the energy it has is proportional to the square of the amplitude. That is to say, when the amplitude of the oscillator is 1, the energy of the oscillator is 1, and when the amplitude is 1.2, the energy is 1.44. When the amplitude is 1.5, the energy is 2.25, and when the amplitude is 2, the energy of the oscillator is 4; it can be deduced by analogy;
In the classical world, we believe that the vibrator can have any amplitude, and the amplitude can be arbitrary, which is continuous. Of course, the energy that the oscillator can have is also continuous.
But Planck now says that amplitude 1.2 and amplitude 1.5 are not allowed, because at this amplitude, the energy of the oscillator is 1.44 and 2.25, which are not integers.
Planck discovered that the release and absorption of energy is discontinuous, just like the white sugar in the supermarket. It is not in bulk, but stacked in bags. You have to buy at least one bag at a time, not half a bag. , You can’t buy one and a half bags, but you can buy two bags, three bags, or four bags.
Then why do we not see in real life that the simple harmonic oscillator can only have a specific amplitude because the energy is part of it? We found that the amplitude is continuous. When we buy white sugar, we also feel that we can buy any amount? This is because Planck’s constant is very small, 37 zeros after the decimal point. Such a small constant cannot be felt by our macroscopic world at all, and it gives us the illusion that the world is continuous.
This day was particularly important on December 14, 1900. It was considered the birth day of quantum theory. On this day, the regular German scientific meeting proceeded as usual, and Planck elaborated on the physics meaning of the black body radiation formula.
What needs special attention here is that Planck only emphasized at the meeting that energy is absorbed and released piece by piece, and the smallest piece is hv, but it never said that the nature of energy is discontinuous. Not to mention that the world is not continuous.
And he also told his colleagues at the meeting, don’t think about it, I never said anything deviant. So when Planck put forward the hypothesis of the amount of energy, all physicists believed that Planck was just a technique used to allow his formula to have an explanation and to give himself a step down.
But when the whole world thought it was a joke, a young man took it seriously. He is Einstein who has graduated, but can’t find a job, and even worry about eating.